An Overview of Pharmaceutical Labeling Requirements
In the labyrinth of pharmaceutical regulations, one element stands above all else—the intricate art of labeling. The label is the bridge between the sterile vial and the safeguarded patient for manufactured drugs. Precision is not just an aspiration; it’s a mandate that ensures compliance with stringent guidelines and, most importantly, safeguards patient health.
Here, we deep-dive into the waves of pharmaceutical labeling, exploring how this seemingly mundane tag is a critical consideration for any pharmaceutical manufacturer, regardless of their geographical setting. Our overview of pharmaceutical labeling requirements will explore the tools and strategies available that ensure compliance without compromising efficiency.
Understanding Pharmaceutical Labeling Regulations
The FDA and International Standards
Pharmaceutical labels are not just about branding; they serve as the regulatory stringency and compliance voice. Each year, the FDA updates pharma labeling rules that shape the practices of stalwart pharma giants and burgeoning industry entrants. These strictures are further fortified by international benchmarks like the European Medicines Agency (EMA) or Japan’s Ministry of Health, Labour and Welfare (MHLW).
Key Labeling Requirements
The essentials of a pharma label are a veritable checklist for patient safety: the drug’s International Nonproprietary Name (INN), the strength of the active ingredient, expiry date, batch number, and manufacturer identity. Besides these overarching demands, there is a need for precise font size and color coding to ensure legibility across different packaging sizes. Other key labeling requirements for pharmaceuticals include:
- Route of administration
- Indication or use
- Contraindications
- Warnings
- Adverse reactions
- Drug interactions
- Date of last update
The Anatomy of Pharma Labeling
Pharmaceutical labeling is a marriage of form and function, each element integral to the product’s overall efficacy. The label must provide accurate and clear information while being durable and legible.
The Crucial Drug Information Panel
The drug information panel is a critical component that demands clarity and precision. Laser marking ensures legibility and durability, which are crucial for storage and preventing errors in healthcare settings.
Dosage Instructions and Regulatory Signposts
Dosage instructions must be clearly delineated with precision, meeting the requirements set forth by regulators. Pharma laser marking has emerged as a quintessential tool for these fine details, consistently etching correct dosages onto each unit.
Warning Labels With No Room for Error
Warnings can be a make-or-break affair for pharmaceutical labels. The gravity of this information necessitates that laser markings aren’t just up to standard but exceed them, ensuring no information erasure or readability issues over time or due to external factors.
Storage Directions for Longevity
Storage instructions are often an overlooked yet vital part of a pharmaceutical label. Laser marking delivers water and chemical-resistant marking, ensuring that the label remains intact for the drug’s shelf life, guiding safety and efficacy.
Regulatory Compliance for Pharmaceuticals
Navigating a Polyglot World
In our increasingly interconnected world, labels often need to communicate in multiple languages. Each new market introduces its nuanced guidelines. Laser marking machines are equipped to deliver this polyglot precision without sacrificing quality or speed.
Serialization and Tracking in a Borderless Industry
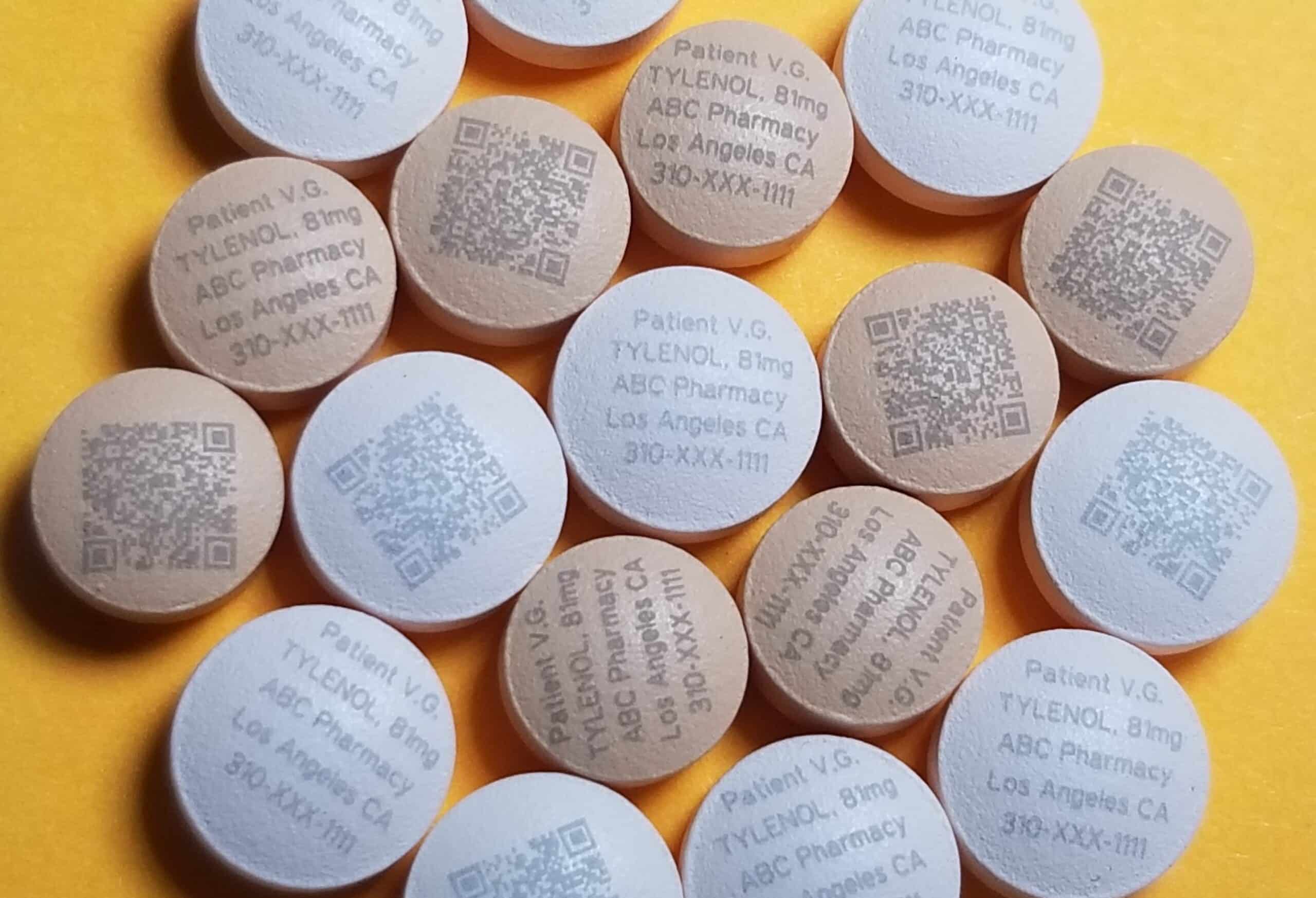
Serialization requirements are on the rise, primarily for the tracking and tracing of pharmaceutical products. Laser marking solutions like those offered by Tri-Star Technologies can now serialize and track products like individual pills, enabling track-and-trace capabilities from production to consumption.
Compliance Challenges in the Labeling Domain
A Moving Target of Changing Regulations
Regulations are in a perennial flux, and pharmaceutical companies must pivot swiftly to adhere to the newest directives. Laser marking provides the ability to update labels with minimal downtime and cost, ensuring compliance without compromise.
Multilingual Labeling
Multilingual labeling can be arduous, especially for those with extensive product lines. Laser marking systems, combined with innovative software, simplify the process of managing multiple sets of information, ensuring that no label is lost in translation.
Serialization
Serialization in the pharmaceutical industry assigns a unique identifier or serial number to each saleable medicine unit. This process enables the tracking and tracing of drugs from production to distribution and ultimately to the patient. It plays a crucial role in combating the global threat of counterfeit medications ensuring patient safety by improving the visibility and security of the supply chain.
Serialization is also where Tri-Star Technologies and our pharmaceutical laser marking machines have been industry leaders for over a decade. The precise and delicate nature of laser marking allows pharmaceutical engineers to laser mark individual pills for unique identification and serialization. These laser marking solutions offer a scalable and efficient way to apply unique markings and create a robust system for combatting counterfeiting.
The Best Practices in Pharmaceutical Labeling
Quality Control
Quality control is the custodian of the label’s integrity. Modern laser marking solutions are equipped with on-machine vision systems that can inspect and ensure the legibility and accuracy of every label, providing an indispensable layer of quality control.
Automation Tools for Accuracy and Efficiency
Automation tools integrated with laser marking systems ensure a reduction in labeling errors, increase productivity, and a streamlined process from creation to application. These systems embody the commitment to precision while upholding the principles of efficacy and efficiency.
Operator Training and Documentation
Training employees to understand the principles and practices of pharmaceutical labeling is paramount. Equipping staff with the necessary knowledge and documentation to understand and operate the labeling systems will further ensure regulatory compliance.
The Science Behind Laser Marking
Laser marking is a noncontact method that uses high-powered lasers to engrave pharma details with high accuracy. The resultant marks are highly durable and legible, making them ideal for pharmaceutical labeling that must withstand time and handling.
High-Contrast Marking
High-contrast marking is a key feature of laser marking technology. This ensures that the labels remain legible despite environmental factors or handling conditions.
Permanent Marking
Laser marking offers permanent and tamper-resistant labeling, making it an invaluable tool in combatting counterfeits and ensuring patient safety.
Clean and Precise Etching
The precision of laser marking delivers clear and concise etching essential for fine print, intricate designs, and barcodes. This precision ensures compliance with regulatory demands while showcasing the company’s commitment to quality.
Find Your Laser Marking Pharmaceutical Labeling Solution at Tri-Star Technologies
Compliance with pharmaceutical labeling requirements is nonnegotiable. It is the ethical and legal standard to which all pharmaceutical manufacturers must adhere, regardless of size or location. Laser marking technology is not merely an option; it’s necessary in the modern pharmaceutical landscape, where precision intersects with patient care. We hope our overview has helped you better understand pharmaceutical labeling requirements and the benefits of laser marking technology.
If you’re searching for a laser marking solution for pharmaceutical labeling, we’re here to help. View our laser marking machines that offer precision, efficiency, compliance, and more. Learn about our machines online or contact our expert staff today.



