How To Select The Best Pharmaceutical Coding Equipment
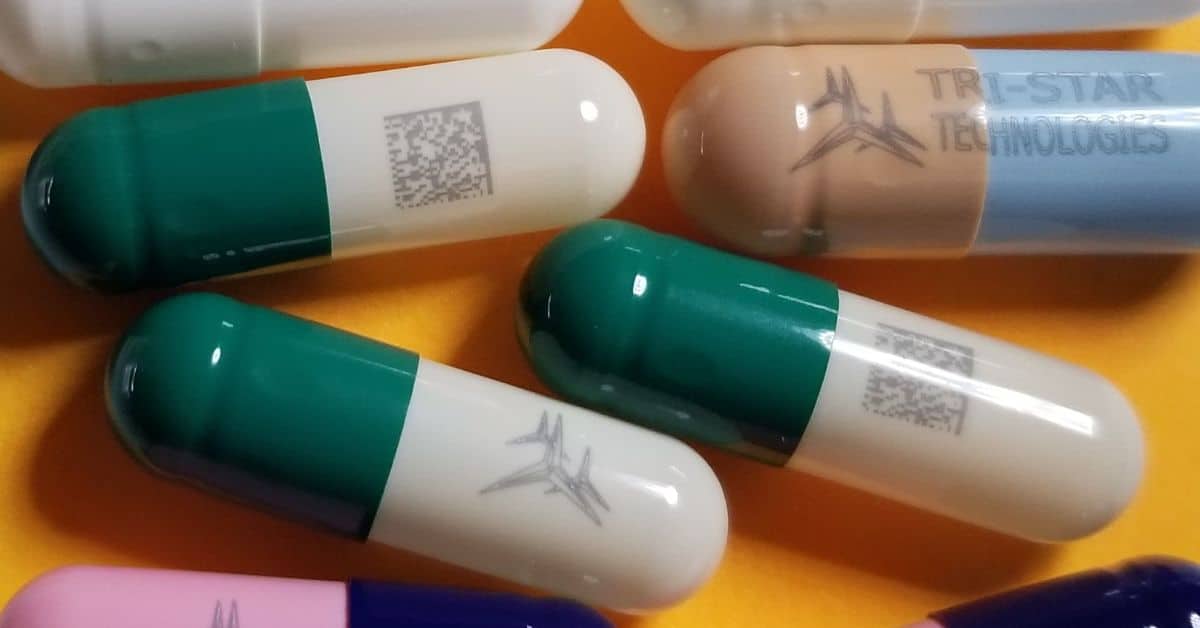
The pharmaceutical industry relies on precise, reliable coding equipment to meet stringent regulatory standards, ensure patient safety, and maintain operational efficiency. With increased emphasis on anti-counterfeit measures and traceability, the right coding equipment is not just a convenience—it’s a necessity.
But how do pharmaceutical manufacturers, quality control managers, and packaging engineers choose the best equipment for their needs? In this guide, we’ll explain the key factors to consider when selecting the best pharmaceutical coding equipment and how Tri-Star Technologies stands out in the industry.
Why Pharmaceutical Coding Is Essential
Pharmaceutical coding serves a dual function: ensuring regulatory compliance and enhancing product traceability across the supply chain. Printed codes on packaging or on tablets provide critical information, such as batch numbers, expiration dates, and unique identifiers. These markings protect against tampering, prevent counterfeiting, and allow quick recalls should issues arise.
Regulatory bodies like the FDA enforce strict requirements regarding code readability, placement, and permanence. Failure to adhere to these standards can result in hefty fines, recalls, and loss of trust among consumers and healthcare providers.
Types of Pharmaceutical Coding Equipment
To meet the diverse needs of pharmaceutical production, several types of coding machines are available. The most common pharmaceutical coding options include inkjet printers, laser marking systems, and thermal transfer overprinters.
1. Inkjet Printers
Pharmaceutical engineers utilize inkjet systems widely due to their speed and ability to handle high production volumes. They are ideal for printing variable data, such as lot numbers or expiration dates, directly onto packaging. While these machines are cost-effective and versatile, their reliance on liquid ink can compromise long-term legibility.
2. Laser Marking Systems
Many manufacturers prefer laser marking thanks to its ability to produce permanent, tamper-resistant codes. These systems emit a focused laser beam that alters the material surface to create durable marks. Unlike inkjet printers, laser markers do not require consumables like ink or ribbons, making them eco-friendly and cost-efficient in the long run.
3. Thermal Transfer Overprinters (TTO)
TTO machines generate high-resolution prints on flexible packaging materials by using heat to transfer ink from a ribbon onto a substrate. While they deliver excellent print quality, they are typically slower than laser systems and best suited for smaller-scale production environments.
Factors To Consider When Selecting Coding Equipment
Selecting the best pharmaceutical coding equipment for your needs isn’t as simple as picking the most affordable option or the fastest machine. Manufacturers must evaluate several key factors to ensure their investment aligns with their production and compliance goals.
Production Volume and Speed
When choosing coding equipment, it’s critical to evaluate daily output requirements and long-term production goals. High-volume manufacturing facilities, for instance, may benefit greatly from laser systems like the M100LPM150 by Tri-Star. This machine is specifically intended for large-scale production, providing the perfect balance of speed and accuracy to meet demanding schedules without compromise.
On the other hand, research labs or smaller operations requiring more compact solutions can turn to models like the M100LPM-R&D, which deliver exceptional precision within a smaller production capacity. Carefully matching the capabilities of your equipment to your production needs will ensure consistent performance and scalability as your operations grow.
Code Legibility and Durability
The integrity and longevity of printed codes are crucial, especially for pharmaceutical products that must maintain traceability throughout their lifecycle. Inkjet printers, although effective for many applications, may fall short when it comes to producing durable marks that can withstand the rigors of transportation, storage, and handling.
Laser marking systems, in contrast, create indelible, permanent marks that neither fade nor smear over time, ensuring compliance and reliability. This durability is particularly important for products requiring extended shelf lives or exposure to varying environmental conditions.
Compliance With Industry Standards
Regulatory compliance isn’t just important—it’s mandatory. Organizations like the FDA, EU FMD, and various other global regulatory bodies govern the pharmaceutical and medical industries with strict standards. Failing to meet these requirements can result in costly recalls, fines, or damage to your brand’s reputation.
Laser marking systems, such as Tri-Star’s Opioid ID Marker, are specifically designed to produce high-quality, tamper-proof codes that adhere to these stringent standards. Their precision and reliability ensure that products meet global compliance requirements, safeguarding both the operation and the end-user.
Integration With Existing Packaging Lines
Integrating new coding equipment into your existing packaging or manufacturing lines can be a complex process, but selecting the right system can make it seamless. Machines with modular designs, customizable features, and user-friendly software—as Tri-Star Technologies offers—simplify integration.
These systems minimize downtime during installation and ensure compatibility with a wide range of production setups. Advanced features like real-time diagnostics and remote support enable smooth transitions and ongoing optimization. By selecting equipment that aligns with your current operations, you can maintain productivity while improving coding efficiency.
Why Tri-Star Technologies Stands Out
In a competitive industry, the pharmaceutical laser marking machines of Tri-Star Technologies stand above the rest. Whether you’re running a high-volume production line or conducting R&D in a lab, Tri-Star offers solutions to your unique challenges.
M100LPM-R&D: Perfect for Small-Scale Production
The M100LPM-R&D model is ideal for small-scale production and research settings. Weighing just 32 pounds, this lightweight, air-cooled machine is easy to transport and versatile enough to mark tablets, capsules, and more—all without compromising the product’s structural integrity.
M100LPM150: Efficiency for Large-Scale Manufacturing
For large-scale manufacturing, the M100LPM150 delivers unmatched efficiency and precision. It can handle high volumes and even mark products through tamper-proof blister packaging, making it the go-to solution for industrial-scale needs.
Opioid ID Marker: A Breakthrough in Pharmaceutical Security
The groundbreaking Opioid ID Marker addresses critical public health challenges like misuse and counterfeiting. This technology embeds unique identifiers within tablets, supporting law enforcement efforts and ensuring medication reaches the intended patient. Its cloud compatibility enables full traceability throughout distribution networks, setting a new standard in pharmaceutical security.
The Benefits of Tri-Star Equipment
Manufacturers worldwide trust Tri-Star Technologies’ solutions for their ability to meet production demands and uphold quality and compliance. Some of the advantages Tri-Star’s laser marking equipment offers to pharmaceutical manufacturers include counterfeit production and production efficiency.
Enhanced Safety and Counterfeit Protection
Tri-Star’s laser systems provide permanent marking solutions that safeguard against unauthorized alterations or counterfeit products. These highly reliable markings ensure both patients and regulators can trust your product’s authenticity and integrity, providing peace of mind in an industry where safety is the priority.
Improved Production Efficiency
Our automated, high-speed laser systems streamline the manufacturing process by eliminating common bottlenecks. These systems maintain exceptional accuracy and robust quality control, even at high production speeds, helping you meet growing demand without compromising standards.
Global Regulatory Compliance
Tri-Star’s equipment meets or exceeds international regulatory standards, simplifying the process of audits and inspections. With these systems in place, you can ensure your operations remain compliant across global markets, reducing risks and maintaining smooth, uninterrupted production.
Transforming Pharmaceutical Safety and Efficiency
The rise of counterfeit drugs and complex regulations calls for innovative solutions in pharmaceutical packaging and coding. Finding the right equipment is essential to protect patient health and boost operational efficiency.
Whether you need a compact solution for a lab or a high-capacity system for industrial production, Tri-Star offers options to meet your needs. Contact our team today for a customized consultation and discover how the right equipment can transform your packaging and coding processes.



