How Pharmaceutical Labeling Regulations Evolved Over Time
Pharmaceutical labeling is one of the most critical aspects of the drug manufacturing and distribution process. Labels provide vital information about the medication, ensure compliance with regulatory requirements, and safeguard public health.
The regulations governing pharmaceutical labeling have evolved over time, adapting to new challenges and technological advancements. Keep reading to learn about the evolution of these regulations and the development of direct UV laser marking.
The Early Days of Pharmaceutical Labeling
In the beginning of the 20th century, pharmaceutical labeling was simple and included the drug’s name, dosage, and basic usage instructions. As the industry expanded, the complexity of medications grew, leading to increased mislabeling, counterfeit drugs, and adverse reactions. These issues prompted regulatory intervention.
The Pure Food and Drug Act of 1906 required the accurate labeling of drug ingredients and effects to protect consumers from harmful or ineffective medications.
Challenges With Initial Regulations
The pharmaceutical industry has faced significant challenges, particularly with counterfeit drugs threatening patient safety. In response, regulatory bodies implemented stricter measures to ensure pharmaceutical labels provided comprehensive information on side effects, contraindications, and storage conditions.
A key development in labeling standards was the 1938 Federal Food, Drug, and Cosmetic Act (FDCA). It required drug manufacturers to demonstrate the safety and efficacy of their products before marketing and mandated detailed usage instructions and risk warnings on labels.
The Rise of Direct Medication Marking
One of the most critical evolutions of pharmaceutical labeling regulations over time occurred in the late 20th and early 21st centuries. One of the most notable advancements during this period was the introduction of direct medication marking through UV laser technology, developed and patented by Tri-Star Technologies. This innovation overcame many of the challenges associated with traditional labeling methods by providing a more precise and long-lasting solution.
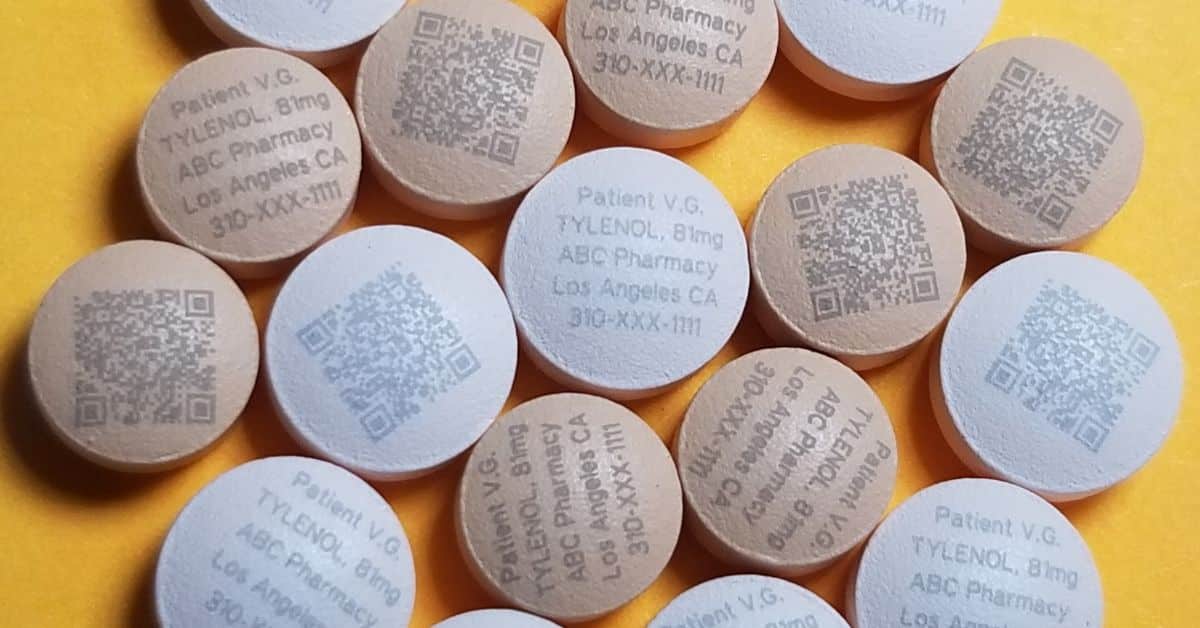
Direct medication marking has revolutionized how pharmaceutical manufacturers apply information, enhancing both functionality and safety. With its ability to produce clear and durable marks directly on the pills and tablets, this technology immensely enhances patient safety and fights counterfeits.
Understanding Direct UV Laser Marking for Pharmaceuticals
Direct UV laser marking utilizes ultraviolet laser radiation to write information directly onto tablets or capsules. Unlike conventional labeling techniques that depend on inks or adhesive labels, this method eliminates the risks of smudging or peeling. The pharmaceutical laser marking equipment interacts with the medication’s surface material, creating a permanent mark without compromising the integrity of the tablet.
To begin, pharmaceuticals coat the tablets with titanium dioxide (TiO2). The UV laser reacts with the TiO2, resulting in a high-contrast mark that remains legible. This method preserves essential details, such as the drug’s name, dosage, and batch number, and enhances security by making it more difficult for counterfeiters to reproduce or alter the information.
Get on the Cutting Edge of Pharmaceutical Technology With Tri-Star
Pharmaceutical labeling has gone from basic labels to sophisticated UV laser marking technology. At Tri-Star Technologies, we’re proud to contribute to this transformation with our cutting-edge direct UV laser marking solutions that combat counterfeiting and maintain legibility throughout a product’s lifecycle. If you’re interested in our innovative technology, learn more about our laser solutions online or contact our expert staff.


